The introduction of the article should aim to captivate the reader’s attention while providing a brief overview of the topic at hand: the type of energy stored in a battery. Batteries play a crucial role in our everyday lives, powering a wide range of devices such as cell phones, laptops, and even electric vehicles. But have you ever wondered what exactly is stored within these small powerhouses? In this article, we will delve into the world of batteries and explore the type of energy they store. From chemical reactions to electrical potential, we will uncover the inner workings of batteries, shedding light on this essential technology that fuels our modern lives.
Inside This Article
- Definition of a Battery and its Importance in Various Industries – Brief Explanation of the Concept of Energy Storage
- Chemical Energy
- Description of how batteries store and release chemical energy
- Explanation of the electrochemical reactions that occur within a battery
- Examples of common types of batteries that store chemical energy (e.g., lithium-ion, lead-acid)
- Electrical Energy
- – Discussion on how batteries convert chemical energy into electrical energy- Explanation of how the flow of electrons generates electrical energy in a battery- Importance of electrical energy storage for portable devices and electric vehicles
- Potential Energy
- – Explanation of how potential energy is stored in certain types of batteries- Discussion on how batteries store energy by separating positive and negative charges- Importance of potential energy storage in rechargeable batteries
- Summary
- Recap of the various types of energy stored in batteries (chemical, electrical, potential) – Emphasis on the significance of battery energy storage in modern technology and daily life
- Conclusion
- FAQs
Definition of a Battery and its Importance in Various Industries – Brief Explanation of the Concept of Energy Storage
A battery is a device that stores and supplies electrical energy through the conversion of chemical energy. It is a portable power source that has become an essential component in numerous industries, providing a reliable and convenient source of energy.
Energy storage is the process of capturing and storing various forms of energy to be used later when needed. Batteries play a vital role in energy storage as they can store and release energy on demand, making them highly versatile and valuable in a wide range of applications.
One of the most significant applications of batteries is in the automotive industry. Electric vehicles (EVs) rely on batteries to store the energy needed to power the vehicle and provide a sustainable alternative to traditional gasoline-powered vehicles. With advancements in battery technology, EVs have become more efficient and can travel longer distances without needing a recharge, revolutionizing the transportation sector.
In the consumer electronics industry, batteries are indispensable. Devices such as smartphones, tablets, laptops, and smartwatches require batteries to function on the go. These portable power sources enable users to carry their devices anywhere, ensuring uninterrupted usage without the need for a constant power supply.
Batteries are also crucial in the renewable energy sector. Solar panels and wind turbines generate energy, but their output can fluctuate depending on weather conditions. By using batteries to store excess energy, it can be stored and deployed when the demand is high or when the primary energy source is not available. This enhances the reliability and stability of renewable energy systems.
In remote areas or during emergencies, batteries serve as a reliable backup power source. They provide energy for critical systems such as healthcare equipment, emergency lighting, communication devices, and more. This ensures that essential services can continue to operate even in challenging circumstances.
Energy storage plays a crucial role in reducing peak demand on the electrical grid. By storing excess energy during periods of low demand and releasing it during times of high demand, batteries help stabilize the grid and prevent power outages. This enhances the efficiency and reliability of the overall energy system.
Overall, batteries are essential in various industries due to their capability to store and supply energy. They enable mobility, provide backup power, enhance renewable energy integration, and ensure a stable energy supply. As technology continues to advance, battery research and development are fundamental in driving innovation and progress in numerous sectors.
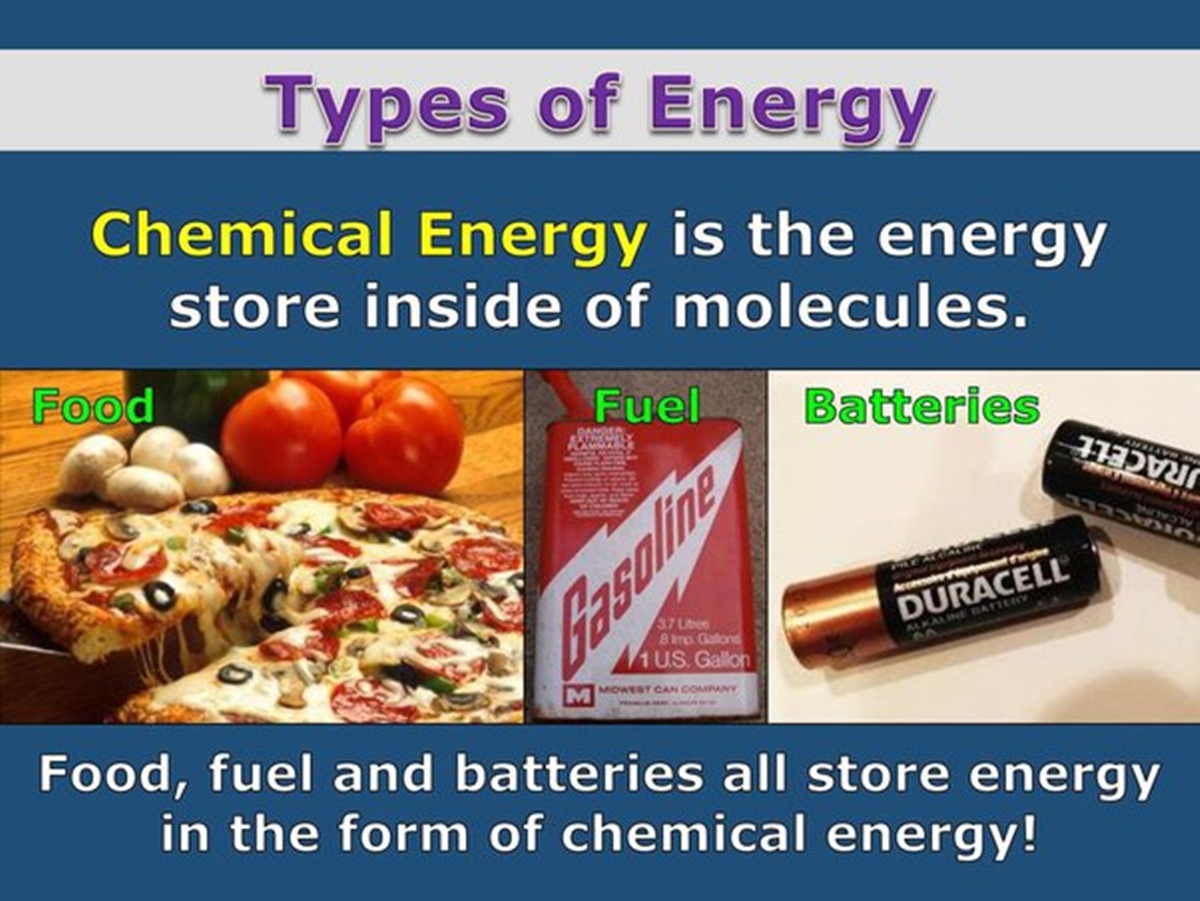
Chemical Energy
Chemical energy is one of the primary types of energy stored in a battery. It is the potential energy that is inherent in the chemical bonds of the battery’s components. During the charging process, this energy is stored in the form of electrochemical reactions that occur within the battery.
When a battery is connected to a circuit and discharging, the chemical reactions inside the battery are triggered, releasing the stored energy. These reactions involve the movement of ions or electrons between different materials within the battery, thereby producing an electric current.
There are various types of batteries that store chemical energy. One commonly used type is the lithium-ion battery, which is popular in portable electronic devices such as cell phones, laptops, and tablets. The lithium-ion battery contains lithium compounds that undergo chemical reactions to release energy.
Another example of a battery that stores chemical energy is the lead-acid battery, commonly found in automobiles. The lead-acid battery uses lead plates immersed in an acid solution to store and release energy through chemical reactions.
Chemical energy storage is crucial in batteries because it allows for efficient and controlled energy release when needed. It also enables rechargeable batteries to be reused by reversing the chemical reactions through the charging process.
Description of how batteries store and release chemical energy
Batteries are ingenious devices that store and release energy in the form of chemical reactions. They are crucial components in various industries, powering everything from cell phones to electric vehicles.
Explanation of the electrochemical reactions that occur within a battery
Within a battery, electrochemical reactions take place to store and release energy. These reactions involve the transformation of chemical substances into different states, resulting in the generation or absorption of electric charges.
During the charging process, chemical compounds in the battery undergo an oxidation reaction at the anode (negative terminal), releasing electrons into the external circuit. At the same time, a reduction reaction occurs at the cathode (positive terminal), which absorbs electrons from the external circuit.
When the battery is discharging, the reverse process takes place. The chemical compounds in the battery undergo a reduction reaction at the anode, capturing electrons from the external circuit. Simultaneously, an oxidation reaction occurs at the cathode, releasing electrons into the external circuit.
Examples of common types of batteries that store chemical energy (e.g., lithium-ion, lead-acid)
There are various types of batteries that store chemical energy. One of the most widely used is the lithium-ion battery. It has a high energy density, making it ideal for portable electronics like smartphones and laptops. Another common type is the lead-acid battery, commonly found in automobiles and backup power systems.
Other examples include nickel-cadmium (NiCd) batteries, which are commonly used in cordless power tools, and nickel-metal hydride (NiMH) batteries, often found in digital cameras and hybrid vehicles. Each type of battery employs different chemical compositions and reactions to store and release energy.
Overall, batteries play a critical role in our daily lives, powering our devices and vehicles. Understanding how they store and release chemical energy through electrochemical reactions can help us appreciate the advancements in modern technology.
Electrical Energy
When it comes to batteries, one of the most important types of energy stored is electrical energy. Batteries are designed to convert chemical energy into electrical energy, which can then be used to power a wide range of devices and equipment. Understanding how batteries store and release electrical energy is key to understanding their functionality and importance in our daily lives.
Within a battery, chemical reactions take place that result in the movement of electrons. These reactions occur between the different materials present in the battery, such as the positive and negative electrodes, electrolytes, and separators. These materials work together to facilitate the flow of charged particles, or ions, between the electrodes.
As these electrochemical reactions take place, a buildup of charges occurs. Specifically, the negative electrode, or anode, becomes negatively charged, while the positive electrode, or cathode, becomes positively charged. This difference in charge creates an electrical potential between the two electrodes, resulting in the storage of electrical energy.
When a circuit is connected to the battery, the stored electrical energy is released. The flow of electrons, from the negatively charged side to the positively charged side, creates an electric current. This current can power various devices and systems, ranging from small electronic gadgets to larger applications like electric vehicles and renewable energy storage.
The ability of batteries to store electrical energy is crucial for the continued advancement of portable electronics and our transition to sustainable energy sources. From smartphones and laptops to electric cars and grid-scale energy storage systems, batteries play a vital role in providing the electrical power we need on a daily basis.
– Discussion on how batteries convert chemical energy into electrical energy- Explanation of how the flow of electrons generates electrical energy in a battery- Importance of electrical energy storage for portable devices and electric vehicles
Batteries play a crucial role in converting chemical energy into electrical energy, making them vital for various applications. One of the key processes involved in this conversion is the flow of electrons within the battery.
When a battery is connected to a circuit, a chemical reaction takes place inside the battery, causing a buildup of electrons on the negative terminal (anode) and a deficit on the positive terminal (cathode). This difference in charge creates a potential difference, or voltage, between the two terminals.
As the circuit is closed, electrons start flowing from the negative terminal to the positive terminal, facilitated by an external load. This flow of electrons generates an electric current, which is the conversion of chemical energy into electrical energy.
The energy stored in the battery is released as the chemical reactions occur between the electrodes and the electrolyte. These reactions produce an excess of electrons at the negative terminal and a deficiency at the positive terminal. By allowing the electrons to flow through the circuit, the battery continues to convert chemical energy into electrical energy.
The ability to store electrical energy efficiently is of utmost importance for portable devices and electric vehicles. In portable devices such as smartphones and laptops, batteries provide the necessary power to fuel their operations. The energy stored in the battery allows these devices to function without the need for a constant connection to a power source.
Similarly, electric vehicles rely heavily on battery technology for energy storage. Advanced batteries, like lithium-ion batteries, offer high energy densities, enabling electric vehicles to travel longer distances on a single charge. The ability to store large amounts of electrical energy in the battery is crucial for the success and viability of electric vehicles as a sustainable transportation option.
Potential Energy
Potential energy is one of the forms of energy that can be stored in a battery. While chemical energy is the primary energy form in most batteries, certain types of batteries also utilize potential energy as a means of energy storage.
In these batteries, potential energy is stored by creating a separation of positive and negative charges. This separation creates a potential difference, also known as voltage, between the two terminals of the battery. This potential difference determines the amount of energy that can be released when the battery is connected to a circuit.
When a circuit is completed and the battery is connected, the potential energy stored in the battery is converted into electrical energy. As the electrons flow from the negative terminal to the positive terminal, they pass through the circuit, providing power to the connected devices or systems.
Rechargeable batteries, such as lithium-ion batteries used in smartphones and laptops, rely on the concept of potential energy storage. When these batteries are connected to a power source, such as a charger, the direction of the current is reversed, allowing the battery to store potential energy once again by separating the charges.
Potential energy storage in batteries plays a vital role in various applications. It enables the use of portable electronic devices for extended periods, as batteries store and release energy as needed. Additionally, in renewable energy systems, potential energy storage in batteries is crucial for storing excess energy generated from sources like solar panels or wind turbines, which can be used during periods of low energy production.
Overall, potential energy storage in batteries is an essential aspect of modern technology. It allows us to power our devices and systems efficiently, providing the flexibility and convenience required in today’s fast-paced world.
– Explanation of how potential energy is stored in certain types of batteries- Discussion on how batteries store energy by separating positive and negative charges- Importance of potential energy storage in rechargeable batteries
Potential energy is a crucial aspect of energy storage in certain types of batteries. It involves the separation of positive and negative charges within the battery, creating a potential difference that can be harnessed to generate electrical energy.
Inside these batteries, potential energy is stored in the form of chemical potential energy. This occurs through the use of electrochemical reactions, where chemical compounds undergo changes in their electron arrangements. As a result, energy is either absorbed or released in the process.
When a battery is charged, the positive and negative charges are physically separated within the battery cells. This separation creates a potential difference between the electrodes, with the positive charges accumulating on one electrode and the negative charges on the other.
During discharge, a pathway is established for the charges to flow between the electrodes, allowing the potential energy to be converted into electrical energy. This flow of electrons generates an electric current that can be utilized to power various devices or systems.
Rechargeable batteries, such as lithium-ion batteries, are designed to enable the storage and release of potential energy multiple times. These batteries have a reversible electrochemical reaction, allowing them to be charged and discharged repeatedly without significant loss of performance.
The importance of potential energy storage in rechargeable batteries cannot be overstated. It enables us to use these batteries in devices such as smartphones, laptops, and electric vehicles, providing a portable and reliable power source. By storing potential energy, rechargeable batteries offer the convenience of being able to recharge and reuse them, reducing the need for single-use batteries and minimizing environmental impact.
Overall, the storage of potential energy within batteries plays a critical role in our modern world. Whether it’s powering our everyday devices or fueling the rise of renewable energy systems, the ability to store and harness this energy is essential for a sustainable and efficient future.
Summary
Batteries also store potential energy by separating positive and negative charges. This allows them to be rechargeable and retain energy for future use. The ability to store energy in different forms makes batteries a crucial component in modern technology and daily life.
Whether it is the lithium-ion battery in your smartphone, the lead-acid battery in your car, or the rechargeable batteries in your laptop, understanding the different types of energy stored in batteries helps us appreciate their impact on our everyday lives. From staying connected to the world through our mobile devices to reducing dependence on fossil fuels with electric vehicles, battery technology continues to advance, enabling a more convenient and sustainable future.
Recap of the various types of energy stored in batteries (chemical, electrical, potential) – Emphasis on the significance of battery energy storage in modern technology and daily life
Throughout this article, we have explored the various types of energy stored in batteries. Let’s recap the main points and emphasize the significance of battery energy storage in our modern lives.
Batteries primarily store and release chemical energy. They utilize electrochemical reactions to convert stored energy into electrical energy. Common types of batteries that store chemical energy include lithium-ion batteries and lead-acid batteries.
When it comes to electrical energy, batteries play a crucial role in converting chemical energy into usable electricity. The flow of electrons within a battery generates electrical energy, making it possible to power our portable devices and electric vehicles.
In addition to chemical and electrical energy, certain types of batteries also store potential energy. This is achieved by separating positive and negative charges, resulting in stored energy that can be released and reused in rechargeable batteries.
The significance of battery energy storage in modern technology cannot be overstated. We rely on batteries to power our smartphones, laptops, tablets, and other portable devices, allowing us to stay connected and productive on the go.
Battery energy storage is also crucial in the automotive industry, enabling the use of electric vehicles and reducing our dependence on fossil fuels. This not only helps to combat climate change but also promotes sustainable transportation options for a greener future.
Furthermore, battery energy storage plays a vital role in backup power systems. In the event of a power outage, batteries can provide emergency power, ensuring that essential systems such as healthcare facilities, data centers, and communication networks continue to function seamlessly.
It’s clear that battery energy storage is integral to our daily lives, powering the devices we rely on and enabling advancements in technology. As the demand for portable devices, electric vehicles, and renewable energy sources continues to grow, the importance of battery technology and energy storage will only increase.
Therefore, ongoing research and development efforts aimed at improving battery performance and sustainability are crucial. From extending battery life and enhancing energy efficiency to exploring alternative materials and recycling methods, there is a constant drive to push the boundaries of battery technology.
Conclusion
In conclusion, batteries store chemical energy and convert it into electrical energy, making them a crucial component in various electronic devices. Understanding the type of energy stored in a battery helps us appreciate the remarkable capabilities of these power sources. From smartphones to laptops, from electric cars to solar-powered homes, batteries have revolutionized the way we live and interact with technology. As our energy needs continue to evolve, so does the development of new battery technologies, aiming to provide more efficient, sustainable, and long-lasting power solutions. So, the next time you use your smartphone or plug in your laptop, remember the incredible energy stored within that compact battery and how it powers your devices in the palm of your hand.
FAQs
1. What type of energy is stored in a battery?
Batteries store chemical energy. This energy is converted into electrical energy that can be used to power various devices such as cell phones, laptops, and flashlights. The chemical reactions happening inside the battery release electrons, creating a flow of electrical charges.
2. How long does a battery last?
The lifespan of a battery depends on various factors, including the type of battery, usage patterns, and the device it powers. Generally, rechargeable batteries can be used for hundreds to thousands of charge cycles before their capacity starts to degrade. On the other hand, disposable batteries have a limited lifespan and need to be replaced once their energy is depleted.
3. How can I extend the battery life of my cell phone?
To extend the battery life of your cell phone, you can follow these tips:
- Reduce screen brightness and timeout settings
- Disable unnecessary background apps and push notifications
- Limit the use of power-intensive features like GPS and Bluetooth
- Enable power-saving mode on your device
- Close unused apps and clear cached data regularly
- Ensure your device software is up to date
- Avoid extreme temperatures, as they can negatively impact battery performance
4. Are all phone chargers the same?
No, not all phone chargers are the same. While most modern smartphones have standardized charging ports, the quality and capabilities of chargers can vary. It’s essential to use a charger that is compatible with your specific device and meets the necessary voltage and amperage requirements. Using low-quality or counterfeit chargers can pose risks to both your device and personal safety.
5. Can I overcharge my phone battery?
Modern smartphones are designed with built-in protection mechanisms to prevent overcharging. Once the battery reaches its full capacity, the charging process stops automatically. However, it’s recommended to unplug your phone once it reaches 100% to avoid unnecessary stress on the battery. Additionally, exposing your phone to extreme heat while charging can degrade the battery over time, so it’s best to avoid such situations.
