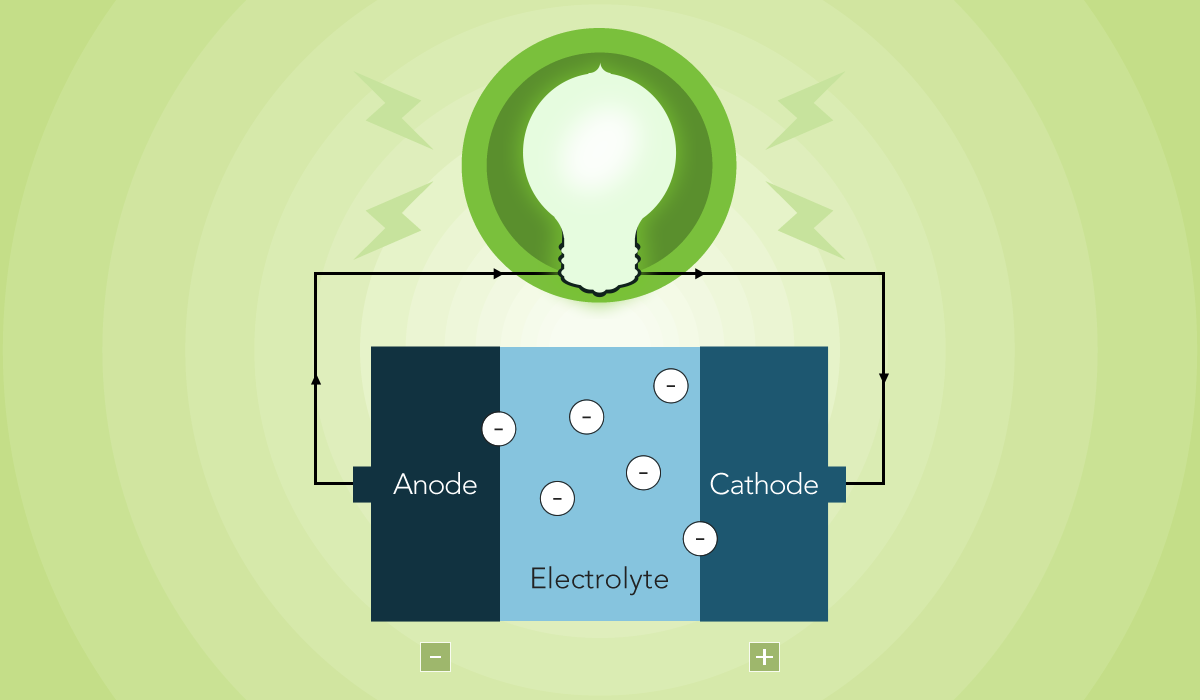
The electrolyte in a battery is a crucial component that facilitates the flow of electrical charge between the battery's cathode and anode. It is typically a liquid or gel substance containing ions, which are electrically charged particles. The electrolyte plays a vital role in the battery's overall performance and efficiency. Understanding the nature of the electrolyte is essential for maximizing the lifespan and functionality of various types of batteries, including those used in mobile devices and other electronic gadgets. In this article, we will delve into the significance of the electrolyte in batteries, its composition, and its impact on battery performance. Let's explore the fascinating world of battery electrolytes and their pivotal role in powering our everyday devices.
Inside This Article
- Electrolyte in a Battery: An Overview
- Types of Electrolytes in Batteries
- Properties of Electrolytes in Batteries
- Conclusion
- FAQs
Electrolyte in a Battery: An Overview
When you think of a battery, the first thing that comes to mind is probably the familiar cylindrical shape or the rectangular block that powers your devices. However, beyond its physical appearance, a battery is a complex system that relies on various components to function effectively. One crucial element within a battery is the electrolyte, which plays a pivotal role in facilitating the flow of ions between the electrodes.
The electrolyte serves as a medium for the movement of ions between the positive and negative electrodes of the battery. This movement is essential for the conversion of chemical energy into electrical energy, enabling the battery to power your electronic devices. Essentially, the electrolyte acts as a conductor, allowing the transfer of ions while maintaining the necessary chemical balance within the battery.
Understanding the role of the electrolyte in a battery provides insight into the fundamental processes that occur within this power source. From its composition to its impact on battery performance, the electrolyte is a critical component that influences the overall efficiency and reliability of a battery.
Types of Electrolytes in Batteries
There are several types of electrolytes used in batteries, each with its own set of characteristics and applications. Some of the most common types include:
Lithium-ion Batteries: These batteries typically use a lithium salt dissolved in a solvent as the electrolyte. This type of electrolyte allows for high energy density and long cycle life, making it popular in portable electronic devices and electric vehicles.
Lead-acid Batteries: Lead-acid batteries commonly use a sulfuric acid solution as the electrolyte. This type of electrolyte is well-suited for starting, lighting, and ignition (SLI) applications in vehicles due to its ability to deliver high current.
Nickel-metal Hydride (NiMH) Batteries: The electrolyte in NiMH batteries is usually a potassium hydroxide solution. NiMH batteries are known for their relatively high energy density and are often used in applications such as hybrid vehicles and rechargeable consumer electronics.
Lithium Polymer Batteries: These batteries utilize a solid or gel-like electrolyte, which offers flexibility in design and reduces the risk of electrolyte leakage. They are commonly used in slim, lightweight devices such as smartphones and tablets.
Nickel-cadmium (NiCd) Batteries: NiCd batteries employ a potassium hydroxide electrolyte. While less common today due to environmental concerns related to cadmium, they were widely used in the past for applications like cordless power tools and emergency lighting.
Each type of electrolyte has its own advantages and limitations, making it crucial to select the appropriate battery type based on the specific requirements of the intended application.
Properties of Electrolytes in Batteries
Electrolytes in batteries possess several crucial properties that directly impact the performance and safety of the battery. These properties play a significant role in determining the efficiency, lifespan, and overall functionality of the battery.
One of the key properties of electrolytes is their conductivity. High ionic conductivity is essential for facilitating the movement of ions between the electrodes, enabling the flow of current within the battery. Additionally, low electronic conductivity helps prevent internal short circuits, contributing to the stability of the battery.
The viscosity of the electrolyte is another vital property. Optimal viscosity ensures uniform distribution of ions within the battery, promoting efficient ion transport and enhancing the overall performance of the battery.
Electrolyte stability is critical for the long-term functionality of the battery. A stable electrolyte minimizes the risk of chemical decomposition, which can lead to capacity loss and reduced cycle life. Moreover, stability contributes to the safety of the battery by preventing thermal runaway and potential hazards.
Furthermore, the compatibility of the electrolyte with electrode materials is essential. A compatible electrolyte ensures proper electrode/electrolyte interface interactions, minimizing side reactions and enhancing the overall efficiency and longevity of the battery.
Lastly, the flammability and volatility of the electrolyte are crucial properties that impact the safety of the battery. Low flammability and volatility reduce the risk of fire hazards, making the battery safer for various applications.
Understanding the electrolyte in a battery is crucial for comprehending its function and performance. The electrolyte, typically a liquid or gel substance, plays a fundamental role in facilitating the flow of ions between the battery’s electrodes, enabling the conversion of chemical energy into electrical energy. As a result, the selection and composition of the electrolyte significantly impact the battery’s efficiency, lifespan, and safety.
With advancements in battery technology, researchers and manufacturers continue to explore innovative electrolyte solutions to enhance energy storage, safety, and environmental sustainability. As the demand for high-performance batteries grows across various industries, the evolution of electrolyte materials and designs will continue to drive progress in energy storage systems, electric vehicles, portable electronics, and beyond.
FAQs
Q: What is an electrolyte in a battery?
A: The electrolyte in a battery is a substance that conducts electricity and allows the flow of ions between the positive and negative electrodes, enabling the chemical reactions that produce electrical energy.
Q: What is the composition of battery electrolytes?
A: Battery electrolytes are commonly composed of salts, solvents, and additives. The salts, such as lithium salts in lithium-ion batteries, are essential for ion conduction, while the solvents facilitate the movement of ions and provide a medium for ion transport. Additives are often included to enhance the performance and safety of the electrolyte.
Q: How does the electrolyte affect battery performance?
A: The electrolyte significantly impacts battery performance by influencing factors such as ion conductivity, temperature stability, and safety. An optimal electrolyte composition is crucial for achieving high energy density, fast charging capabilities, and long-term durability in batteries.
Q: Can the electrolyte in a battery be replaced or replenished?
A: In certain types of batteries, such as lead-acid batteries, the electrolyte can be replenished by adding distilled water or a specific electrolyte solution. However, in sealed or maintenance-free batteries like lithium-ion batteries, the electrolyte cannot be easily replaced due to the sealed design.
Q: What are the safety considerations related to battery electrolytes?
A: Safety considerations regarding battery electrolytes include their flammability, reactivity with moisture or air, and potential for leakage. It is crucial to handle and store batteries with care, especially when dealing with electrolytes, to prevent accidents and ensure safe operation.
