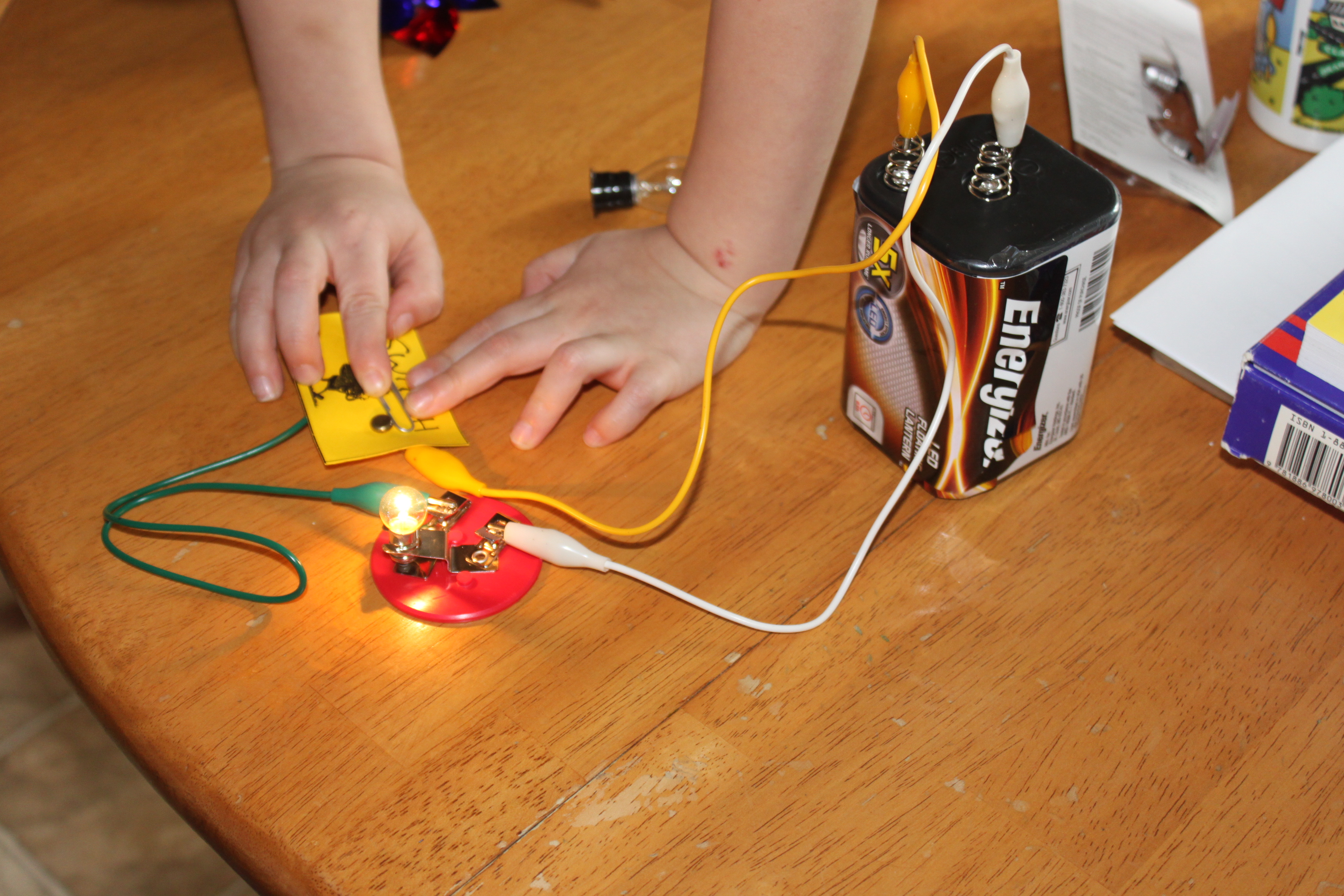
When it comes to understanding the inner workings of a battery, one question that often arises is: What creates an electric current in a battery? To answer this, we delve into the realm of electrochemical reactions. Within a battery, there are two electrodes – a cathode and an anode – immersed in an electrolyte solution. These electrodes are made of different materials, with the cathode typically being a metal oxide and the anode composed of a metal or a carbon-based material. When the battery is connected to an external circuit, a chemical reaction takes place at the electrodes, leading to the creation of an electric current. This chemical reaction involves the movement of electrons between the anode and the cathode, facilitated by the flow of ions through the electrolyte. Understanding this process is crucial for comprehending the functioning of batteries and the role they play in powering our modern-day devices.
Inside This Article
- What is an Electric Current?
- Components of a Battery
- Chemical Reactions Inside a Battery
- Role of Electrolytes in Current Generation
- How Electrons Flow in a Battery
- Factors Affecting Electric Current in a Battery
- Conclusion
- FAQs
What is an Electric Current?
An electric current is the movement or flow of electrically charged particles, typically electrons, through a conducting medium. It is a fundamental concept in electricity and is essential for the operation of electrical devices. Essentially, electric current refers to the flow of energy caused by the motion of electrons in a closed circuit.
The flow of electric current can be compared to the flow of water in a pipe. In a water pipe, the water molecules move from one end to the other, creating a continuous flow. Similarly, in an electric circuit, electrons move along a path, creating an electric current.
The unit of measurement for electric current is the ampere (A). One ampere is defined as the flow of one coulomb of charge per second. Coulomb is the unit of electric charge, and it represents the amount of charge carried by a specific number of electrons.
Electric current can flow in two forms: direct current (DC) and alternating current (AC). In direct current, the flow of electrons is in one direction, like a steady stream. This type of current is commonly found in batteries and most electronic devices. On the other hand, alternating current reverses its direction periodically, typically at a frequency of 50 or 60 hertz. AC is the type of current that is used to power homes and businesses.
Components of a Battery
A battery is a self-contained power source that converts chemical energy into electrical energy. It consists of several key components, each playing a crucial role in the functioning of the battery. Understanding the components of a battery can provide valuable insights into how it generates and delivers electric current.
1. Anode: The anode is the negative terminal of the battery and is where oxidation takes place. It is typically made of a metal or alloy that readily donates electrons during the chemical reactions within the battery.
2. Cathode: The cathode is the positive terminal of the battery and is responsible for the reduction reactions. It is usually made of a different material than the anode and is designed to receive electrons from the anode.
3. Electrolyte: The electrolyte serves as a medium for ion flow between the anode and the cathode. It can be a liquid, gel, or solid substance that contains ions and facilitates the movement of charged particles within the battery.
4. Separator: The separator is a porous material placed between the anode and the cathode to prevent direct contact between them. It allows the flow of ions while preventing the formation of short circuits within the battery.
5. Collector: The collector is responsible for collecting the current generated from the chemical reactions in the battery and delivering it to an external circuit. It is usually made of conductive materials such as copper or aluminum.
6. Terminal: The terminals are the points on the battery where external connections can be made. They allow the battery to be connected to devices or circuits, enabling the transfer of electric current.
Each of these components plays a vital role in the overall performance of the battery. The chemical reactions occurring between the anode and cathode, facilitated by the electrolyte, result in the generation of electric current. The separator prevents any unwanted electrical contact, while the collector and terminals ensure that the current can be utilized in external devices.
Understanding the components of a battery helps us appreciate the intricate process of how chemical energy is converted into the electrical energy that powers our electronic devices.
Chemical Reactions Inside a Battery
When we talk about the chemical reactions inside a battery, we are referring to the processes that convert chemical energy into electrical energy. Batteries consist of two or more electrodes, an electrolyte, and a separator. These components work together to facilitate the chemical reactions necessary for the generation of electric current.
Inside the battery, there are two electrodes: a cathode and an anode. The cathode is the positive terminal and the anode is the negative terminal. These electrodes are made of different materials, which play a vital role in the battery’s overall function.
One of the most common types of batteries, the alkaline battery, utilizes a chemical reaction between a zinc anode and a manganese dioxide cathode. As the battery discharges, zinc ions react with hydroxide ions from the electrolyte to form zinc oxide and water. Simultaneously, the manganese dioxide releases oxygen atoms, which combine with zinc ions to form zinc oxide. These reactions generate electrons at the anode, which flow through an external circuit to the cathode, creating an electric current.
Another type of battery, the lead-acid battery, involves a chemical reaction between a lead anode and a lead dioxide cathode. The electrolyte in this battery is a solution of sulfuric acid. As the battery discharges, the lead at the anode reacts with sulfuric acid to form lead sulfate and hydrogen ions. The lead dioxide at the cathode also reacts with sulfuric acid, producing lead sulfate and oxygen ions. These reactions result in the conversion of chemical energy into electrical energy.
It’s worth noting that different types of batteries utilize different chemical reactions. Lithium-ion batteries, for example, utilize lithium compounds as the active materials in their electrodes. These compounds undergo oxidation and reduction reactions, releasing lithium ions and electrons, which flow through the external circuit to generate electric current.
The chemical reactions inside a battery are essential for the continuous generation of electrical energy. Understanding these reactions gives us insight into how batteries work and how they are able to power our devices and equipment efficiently.
Role of Electrolytes in Current Generation
Electrolytes play a crucial role in the generation of electric current within a battery. An electrolyte is a substance that conducts electricity when it is dissolved or melted. It acts as a medium for the movement of ions between the battery’s electrodes. In most batteries, the electrolyte is typically a liquid or gel-like substance.
The electrolyte contains ions that are responsible for the flow of electric charge. These ions can be either positively charged (called cations) or negatively charged (called anions). When a battery is connected to a circuit, a chemical reaction occurs within the battery, causing the cations and anions to move towards their respective electrodes.
During the discharge process, the electrolyte facilitates the flow of ions from the negative electrode, also known as the anode, towards the positive electrode, known as the cathode. This movement of ions creates a flow of electric charge, allowing the battery to power devices.
Moreover, the electrolyte helps maintain the balance of charge within the battery. As the cations and anions move towards their respective electrodes, electrons are produced at the anode and consumed at the cathode. This movement of electrons maintains the electric current throughout the battery.
It is important to note that different types of batteries require different types of electrolytes. For example, lead-acid batteries commonly used in automotive applications have a sulfuric acid electrolyte. Lithium-ion batteries, on the other hand, use a non-aqueous electrolyte composed of lithium salts.
The characteristics of the electrolyte, such as its conductivity and stability, can significantly impact the performance and lifespan of a battery. An optimal electrolyte enhances the efficiency and reliability of current generation within the battery.
How Electrons Flow in a Battery
Understanding how electrons flow in a battery is essential to comprehend the working principle of this power source. When a circuit is complete, a pathway is created for electrons to flow from the negative terminal of the battery to the positive terminal. This flow of electrons is what we refer to as an electric current.
Inside a battery, there are two terminals: the positive terminal, also known as the cathode, and the negative terminal, known as the anode. These terminals are connected by an electrolyte, which is a chemical substance that facilitates the flow of electrons.
When a battery is connected to a circuit, a chemical reaction occurs within the battery. The anode undergoes an oxidation reaction, releasing electrons into the circuit. These electrons then flow through the external circuit, delivering electrical energy to power devices or perform work. Meanwhile, the cathode undergoes a reduction reaction, accepting the electrons back from the circuit.
This flow of electrons from the anode to the cathode creates the electrical current. It is important to note that it is the movement of electrons, not the movement of the entire battery itself, that generates the current.
The movement of electrons in a battery can be visualized as a closed loop circuit. The negative terminal of the battery repels the electrons, pushing them towards the positive terminal. As the electrons travel through the circuit, they provide energy to power devices, such as cell phones or flashlights.
It is crucial to mention that the electrolyte plays a significant role in facilitating the electron flow. The electrolyte allows the ions within it to move freely, enabling the transfer of charge between the anode and cathode. Without the presence of an electrolyte, the flow of electrons would be hindered, preventing the battery from generating an electric current.
It’s worth noting that the flow of electrons in a battery is not endless. Eventually, the chemical reaction within the battery will deplete the supply of reactants, reducing the flow of electrons and causing the battery to lose its ability to produce a current. This is when the battery needs to be recharged or replaced to restore its functionality.
Factors Affecting Electric Current in a Battery
Electric currents play a vital role in powering various devices, and batteries are the primary source of this electrical energy. However, several factors can impact the flow of electric current within a battery. To better understand these factors, let’s delve into the intricacies of how they affect the operation of a battery.
1. Battery Chemistry: Different battery chemistries have varying capabilities to generate electric current. For example, lithium-ion batteries are commonly used in smartphones due to their high energy density and ability to sustain a steady current flow. On the other hand, lead-acid batteries, commonly found in automobiles, provide a large initial surge of current but are less efficient for prolonged use.
2. Battery Capacity: The capacity of a battery refers to its ability to store and deliver electrical energy. A higher capacity battery can supply a larger amount of current over a longer period. This is vital in applications where a device requires a sustained and continuous flow of electricity.
3. Battery Age and Condition: As batteries age, their ability to generate and sustain a current diminishes. Factors such as temperature, usage patterns, and charging practices can impact a battery’s overall health and performance. Aging batteries may exhibit reduced capacity and an increased internal resistance, leading to a decrease in the available current.
4. Temperature: Temperature has a significant influence on a battery’s performance. Extreme temperatures, whether too hot or too cold, can affect the chemical reactions inside the battery and alter its ability to generate current. High temperatures can accelerate chemical reactions, shortening the battery lifespan, while low temperatures can decrease the overall battery capacity.
5. Load Resistance: The resistance offered by the connected device, also known as load resistance, affects the flow of current from a battery. Higher load resistance restricts the current flow as it creates more resistance for the electrons to overcome. Conversely, lower load resistance allows for a higher current flow.
6. Internal Resistance: Every battery has an internal resistance that impacts the overall current output. This resistance is influenced by factors such as battery chemistry, construction, and temperature. Higher internal resistance results in a voltage drop within the battery, reducing the available current to the connected device.
7. Charging and Discharging Rates: The rate at which a battery is charged or discharged can impact its ability to provide an adequate current. Rapid charging or discharging can increase the internal temperature of the battery, affecting its performance and potentially reducing the available current.
By understanding the factors that affect electric current in a battery, we can make informed decisions about battery usage, maintenance, and selection for specific applications. It is crucial to consider these factors to ensure optimal performance and longevity of devices powered by batteries.
Conclusion
In conclusion, the creation of an electric current in a battery is a fascinating process that involves the conversion of chemical energy into electrical energy. This conversion is made possible by the chemical reactions taking place within the battery, which involve the movement of electrons from the negative terminal to the positive terminal.
As we have discussed, batteries consist of two electrodes, an electrolyte, and a separator. The chemical reactions occurring between the electrodes and the electrolyte allow for the transfer of electrons, generating an electric current. The specific chemistry of the battery determines its voltage, capacity, and lifespan.
Understanding how batteries produce electrical energy is crucial for various applications, from powering everyday electronics to providing energy storage for renewable sources. As technology advances, the development of more efficient and sustainable battery technologies remains a critical area of research.
By knowing the inner workings of batteries, we can make informed choices when selecting and using mobile accessories such as power banks, chargers, and portable battery packs. So, the next time you charge your phone or use any electronic device, remember the remarkable process that occurs within the battery to keep your devices powered up and running.
FAQs
Q: How does a battery create an electric current?
A: A battery creates an electric current through a chemical reaction that occurs inside it. When a battery is connected to a circuit, the reaction between its positive and negative terminals produces a flow of electrons, which creates the electric current.
Q: What is the chemical reaction that takes place inside a battery?
A: The chemical reaction inside a battery typically involves the movement of ions between the positive and negative terminals. In a typical alkaline battery, for example, the reaction involves zinc and manganese dioxide. Zinc ions combine with hydroxide ions to form zinc oxide, while manganese dioxide reacts with hydrogen ions to form water.
Q: What are the different types of batteries?
A: There are various types of batteries available, including alkaline batteries, lithium-ion batteries, lead-acid batteries, nickel-cadmium batteries, and nickel-metal hydride batteries. Each type has its own characteristics and is suitable for different applications.
Q: How long does a battery last?
A: The lifespan of a battery depends on various factors such as its type, capacity, and usage. Generally, batteries can last anywhere from a few hours to several years. Factors like the number of charging cycles, operating temperature, and storage conditions can also affect a battery’s lifespan.
Q: Can I recharge a non-rechargeable battery?
A: No, non-rechargeable batteries, also known as primary batteries, are not designed to be recharged. Attempting to recharge them can be dangerous and could lead to leakage, overheating, or even explosion. It’s important to use the appropriate type of battery for your device and follow the manufacturer’s instructions.
